NeuroGenesis plans to initiate randomized double-blind Phase IIb studies in secondary progressive MS & ALS. Clinical sites include Mass General, Brigham & Women’s Hospital, University of Miami, NYU Langone and Hadassah Medical Center. Each clinical site will be managed by world renowned KOLs.
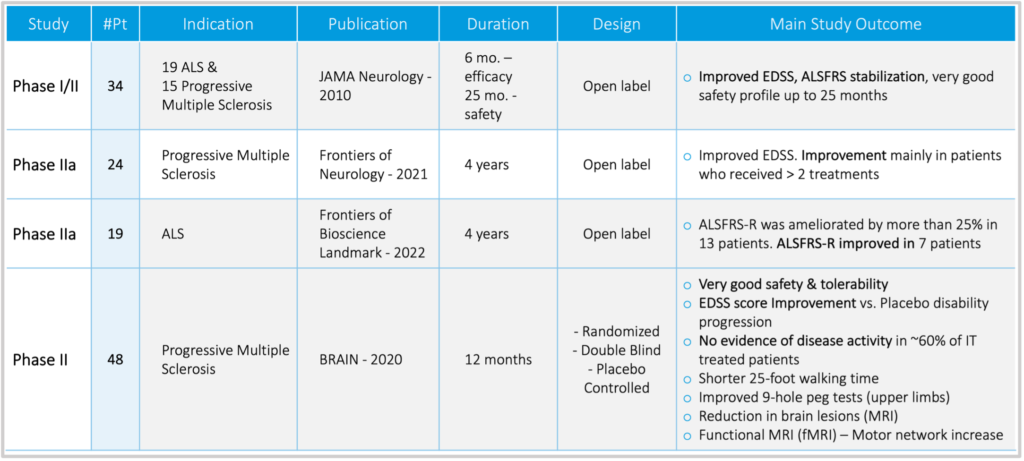
Following dozens of pre-clinical studies which resulted in over 20 pre-clinical publications, NG01 was administered in a Phase IIa (NCT00781872) open label trial to 34 progressive MS and ALS patients. The results were quite encouraging as the mean ALSFRS score remained stable whereas the mean EDSS score improved. There were no serious treatment-related adverse events during the duration of the study.
In a Phase IIb (NCT04823000) open-label long-term study in 24 progressive MS patients, the repeated administration of NG01 for a period of up to four years showed significant clinical benefits (improved disability, EDSS scores and immunomodulatory effects) particularly in patients treated with >2 injections. There were no serious treatment-related adverse events during the whole four-year duration of the study.
The randomized Phase 2 trial (NCT02166021) was a 48-patient double-blind study that
compared clinical effects of intravenous (IV) and intrathecal (IT) injections of NG-01 with
those of sham injections in patients with active or worsening progressive MS. The study
duration was 14 months. No serious treatment-related safety issues were reported in this
study.
Significantly fewer patients experienced treatment failure in the NG-01 IT (intrathecal
administration) and NG-01 IV (intravenous administration) groups compared with those in
the sham-treated group (6.7%, 9.7%, and 41.9%, respectively, p = 0.0003 and p = 0.0008).
During the one-year follow-up, 58.6% and 40.6% of patients treated with NG-01 IT and NG-
01 IV, respectively, exhibited no evidence of disease activity compared with 9.7% in the
sham-treated group (p< 0.0001 and p< 0.0048, respectively). NG-01 IT transplantation
induced additional statistically significant benefits on the relapse rate, on the monthly
changes of the T2 lesion load on MRI, and on the timed 25-foot walking test, 9-hole peg test,
optical coherence tomography, functional MRI, and cognitive tests.
Dorit Harati brings over 20 years of experience as a Regulatory & Quality Assurance for cell therapy companies in Israel, EU and the US either as a consultant or as a member of the management team. She is an expert on Quality Assurance, Quality Management Systems (QMS), CMC, product development, aseptic and cGMP manufacturing, facility design, cross-continental technology transfer, all in accordance with the EU and FDA regulations.
Before joining Neurogenesis, Dorit was a senior consultant at Dark Horse Consulting Group, a consulting firm that provides comprehensive services to Cell & Gene Therapy companies in the US, EU and Asia. Previously, she has also served as VP Quality Assurance, Manufacturing and Logistics at Gamida Cell, where she established and managed the Quality Assurance, Quality Control, Logistics and Manufacturing Departments, technology transfer to different CROs.
Mark Freedman brings more than 30 years of expertise as a leading neurologist and neuroimmunology scientist running therapeutic trials in neurodegenerative diseases. He also has an extensive background in researching the cellular mechanisms behind the immune-mediated demyelination associated with multiple sclerosis.
He holds several prestigious leadership positions, some of which include President of ACTRIMS (Americas Committee for Treatment and Research In Multiple Sclerosis), Director of the Multiple Sclerosis Research Unit (Neurology) at the Ottawa Hospital General Center, and Professor of Medicine (Neurology) at the University of Ottawa.
Dr. Netzer served for over 15 years in cell therapy companies in Israel and in the US as a member of the management team. He is an expert in Stem Cell R&D and operations and in establishing a global strategy for product development, manufacturing, and supply chain, with a clear focus on market viability. Before joining NeuroGenesis, Dr. Netzer served as the VP CMC and Operations at Cell
Cure Neurosciences, acquired by Lineage Cell Therapeutics, Inc. (NYSE American and TASE: LCTX), where he led the development of a novel ‘Thaw- and-Inject’ cell product and the building of Lineage’s state of the art GMP manufacturing facility. Previously, Dr. Netzer was the Research Manager at Pluristem Therapeutics Inc. (NASDAQ: PSTI), where he led the preclinical trials and the finding of the first cell therapy product for treating radiation syndrome following nuclear disasters. Dr. Netzer was a Post-Doctoral fellow in cell biology and viral immunology at the NIAID, NIH and holds a PhD in Biotechnology and immunology from the Technion in Israel.
Dr. Kassis is one of the world’s experts in Regenerative Medicine and stem-cells based technologies, and production of clinical-grade stem cells for treating multiple sclerosis andamyotrophic lateral sclerosis patients. Dr. Kassis has over 20 years of experience in developing innovative cellular therapies from bench to patient bedside. Dr. Kassis serves as a Senior Scientist at the Department of Neurology, Hadassah Medical Center. Dr. Kassis has published over 30 publications in prestigious peer reviewed publications.
Dr. Karussis is considered one the world experts in clinical applications of Stem Cells in Neurological diseases, he is the director of MS Center and department of Neuroimmunology, Cell Therapies at Hadassah Medical Center, Jerusalem (since 2007). Dr. Karussis servers on many editorial boards including the International Multiple Sclerosis, Journal of Neurological Sciences, World Journal of Neurology and others. Dr. Karussis has participated in numerous scientific committees at ECTRIMS, EFNS, ISNI and the European School of Neuroimmunology. Dr. Karussis also serves as the president of the Israeli Neuroimmunology Society (since 2010) and has published over 120 peer-reviewed scientific papers mainly in the field of Stem Cells and Neuroimmunology.
An accomplished senior executive with over 16 years of CEO experience in public and private companies in Tel Aviv, New York and Silicon Valley. Established and directly managed global high growth sales, business development organizations in North America, Europe and Asia (direct Sales, Inside Sales, One Tier, Two-Tiered Distribution, OEM, IP Licensing etc.).
CEO of AppDome, a recognized market leader in mobile integration as a service (security, connectivity, mobility). Recognized by CIO magazine as one of 2015’s Top 10 Mobility CEOs as well as a 2015 Top Mobile Innovator by the Channel Company. President and CEO of INKSURE Technologies, the industry leader in cloud-based mobile product authentication. Winner of the 2011 Prestigious ID People Americas Award, the Oscars of the ID Industry, for his contribution to the high-security authentication market. Proven track record in the software, hardware industry of increasing top and bottom-line results, previously heading SanDisk Enterprise (NASDAQ: SNDK) in North America and M-System (NASDAQ: FLSH) Enterprise business. In 2000, founded and served as CEO of Kavado Inc. (acquired by Protegrity Inc.), a global web security solutions provider.
Tal holds an MBA from Columbia Business School in New York and a BA with honors in Economics, Business Administration from the Hebrew University in Jerusalem.